How Do You Know Which Ideal Gas Constant to Use
Use the ideal gas law and related gas laws to compute the values of various gas properties under specified conditions. Lastly the constant in the equation shown below is R known as the the gas constant which will be discussed in depth further later.

Chem Stuff Medical School Inspiration Basic Physics Physical Chemistry
Also many kinds of research and studies have been going on based on this.

. Recall that the ideal gas equation is given as. R 008206 L atm mol K. The ideal gas law does not make an assumption about the specific heat of a gas.
The ideal gas law is written for ideal or perfect gases. PV nRT P pressure V volume n moles of gas R universal gas constant T temperature. Where is the specific gas constant for a particular gas in units Jkg K and ρ mV is density.
Consider the following equation. In the most general case the specific heat is a. While the ideal gas law can still offer an.
Well we would come soon to that before we wind up this discussion. To use the formula for a real gas it must be at low pressure and low temperature. Increasing pressure or temperature raises the kinetic energy of the gas and forces the molecules to interact.
And when they dont meet the work requirements we replace them. During the seventeenth and especially eighteenth centuries driven both by a desire to understand nature and a quest to make balloons in which they could fly Figure 1 a number of scientists established the relationships between the macroscopic physical. We can rearrange this equation in terms of moles n and then solve for its.
You can use values for real gases so long as they act like ideal gases. Only to be taken advantage of by our employees constant use of their cell phones every waking moment. Do the math and you can put a LOT of gas into your 81 and still be money ahead with performance in the same ballpark.
If we set up the ideal gas law for the values of 1 mole at Standard temperature and pressure STP and calculate for the value of the constant R we can. The ideal gas law uses the formula PV nRT where P is the pressure in atmospheres atm V is the volume in liters L n is the number of moles mol and T is the temperature in kelvin K. Ideal Gas Law The findings of 19th century chemists and physicists among them Avogadro Gay-Lussac Boyle and Charles are summarized in the Ideal Gas Law.
PVnRT Another way to describe an ideal gas is to describe it in mathematically. Since its inception the ideal gas law has been finding its uses in many appliances that we come across in our day to day life. It is an addiction to many people young and old.
DfracPVnRT1 The term fracpVnRT is also called the compression factor and is a measure of the. This isnt really designed to coat the outside of the chain plates which while only cosmetic can get a bit rusty if you havent got the delivery just so. FYI my 05 duramax towing a 612 enclosed trailer w maybe 1K-lbs gear CO-VA 80mph only could manage 11 mpg.
Still though they really do keep the rollers well lubed with little hassle. Whether youre studying for tests or just want to expand your knowledge of science these flash cards are the handy aids you need to learn just about everything you need to know about Dmitri Mendeleevs groundbreaking Periodic Table. I have learned over the years that we need to be fair but firm with what we expect out of our employees.
Applications of Ideal Gas law in real life. Worksheet 7 - Ideal Gas Law I. Now do you know how to calculate the values of ideal gas law variables.
If you have a. Whether you agree or not. Included is a leaflet showing the whole Periodic Table and how to read it plus lots of ideas for games to play.
How do you calculate the molar mass of a gas if you know mass pressure volume and temperature of the gas. There are other chain oilers available too. Whether you just want to have.
This notation is the gas dynamicists version which is more practical in modeling of gas flows involving acceleration without chemical reactions. The testing of Scottoil showed it worked very well when you remember that its a constant loss system. The value of R varies with the units chosen.
If we know mass pressure volume and temperature of a gas we can calculate its molar mass by using the ideal gas equation.

Ideal Gas Constant Easy Science Gas Constant Ideal Gas Law Chemical Changes
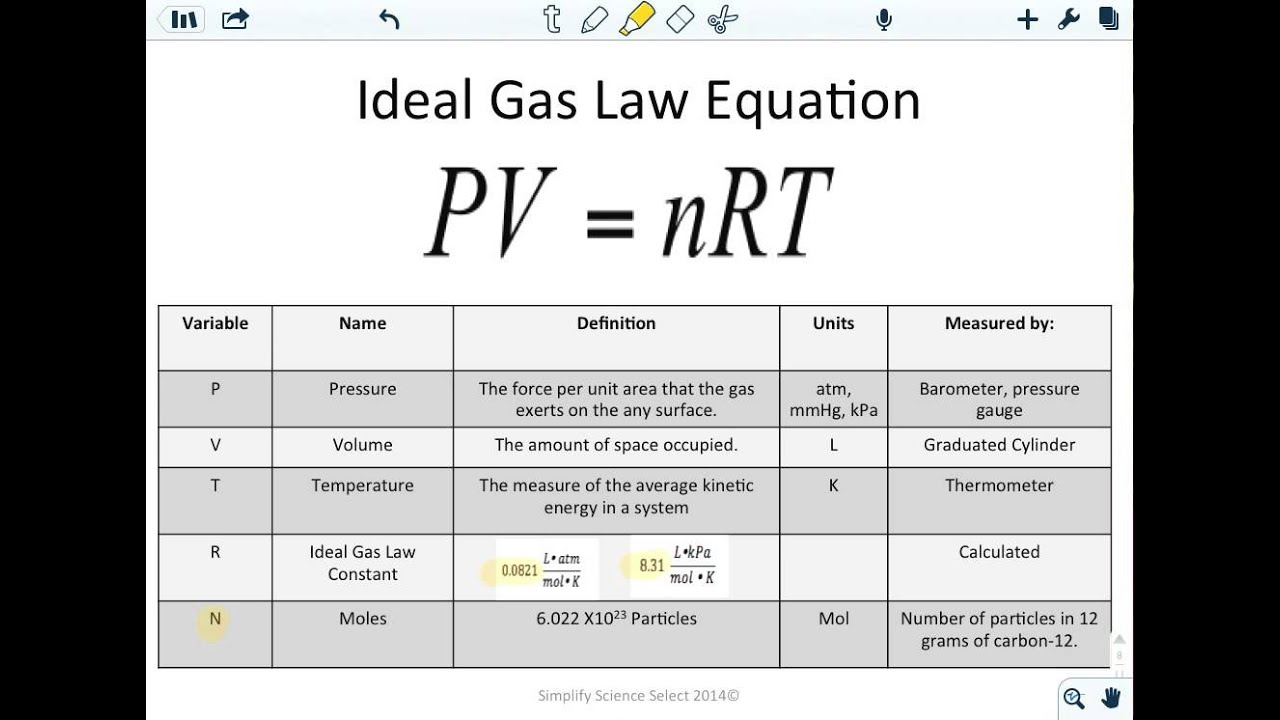
Pin By Jessica Joyce On Ideal Gas Law Ideal Gas Law Gas Laws Chemistry Chemistry Lessons

Ideal Gas Equation Its Derivation Ideal Gas Law Gas Constant Study Chemistry

No comments for "How Do You Know Which Ideal Gas Constant to Use"
Post a Comment